Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kaito, Takeji
JNC TN9400 2000-039, 19 Pages, 2000/03
The corrosion behavior of core materials in lead cooled reactor was investigated as the feasibility study for fast breeder reactor. The results are summarized as follows. (1)The corrosion of stainless steels under lead and lithium occurs mainly due to the dissolution of nickel. Consequently ferritic stainless steels have better resistance to corrosion under lead and lithium than austenitic stainless steels, and the corrosion resistance of high nickel steels is worst. (2)The dissolution rate, D(mg/m /h), is correlated with lead and lithium temperature, T(K), as log
/h), is correlated with lead and lithium temperature, T(K), as log Da = 10.7873 - 6459.3/ T and log
Da = 10.7873 - 6459.3/ T and log Df = 7.6185 - 4848.4/T, where D a is the dissolution rate for austenitic steels and D f is for ferritic steels. lt's possible to calculate the corrosion thickness, C(
Df = 7.6185 - 4848.4/T, where D a is the dissolution rate for austenitic steels and D f is for ferritic steels. lt's possible to calculate the corrosion thickness, C( m), using the following correlation: C = (D
m), using the following correlation: C = (D t)/
t)/
 10
10 , where t is exposure time(hr) and
, where t is exposure time(hr) and  is density of the core matelial (g/cm
is density of the core matelial (g/cm ). (3)The corrosion thickness estimated for austenitic steels using above correlations was extremely larger than ferritic steels, about 6 times at 400
). (3)The corrosion thickness estimated for austenitic steels using above correlations was extremely larger than ferritic steels, about 6 times at 400 C and more than 20 times at above 600
C and more than 20 times at above 600 C. lt's considered that applicable temperature in lead cooled reactor core is below 400
C. lt's considered that applicable temperature in lead cooled reactor core is below 400 C (about 60
C (about 60 m corrosion thickness after 30000 hr) for austenitic steels, and below 500
m corrosion thickness after 30000 hr) for austenitic steels, and below 500 C (about 80
C (about 80  m after 30000 hr) for ferritic steels.
m after 30000 hr) for ferritic steels.
Mizuta, Shunji; ;
JNC TN9400 2000-032, 38 Pages, 2000/03
lt is necessary for feasibility study of fast reactor to evaluate the oxidation of the austenitic stainless steels in the case of using for core material in carbon dioxide gas-cooled reactor. The properties for oxidation of austenitic stainless steels in carbon dioxide were surveyed in literatures and the data were selected after evaluation of factors for oxidation in carbon dioxide. The equation of oxidation in carbon dioxide for PE16, 20Cr/25Ni/Nb, 18Cr-8Ni and JNC Cladding materials were proposed. The equation for oxidation of austenitic stainless steels were expressed as upper limit for the equation according to parabolic law. The equation for JNC cladding materials (PNC316, PNC1520, 14Cr-25Ni) was proposed based the oxidation behavior of 18Cr-8Ni which is same oxidation region for weight gain in three-component system of Fe-Cr-Ni, in addition to evaluate of effect for silicon content. The oxidation equation of 20Cr/25Ni/Nb was applied to the high Ni alloy of JNC cladding material. The obtained equation is as follows, X = 4.4W 1000, W =
1000, W =  , kp =
, kp =  exp(-Q/(RT)), X: oxide thickness[
exp(-Q/(RT)), X: oxide thickness[ m], W : weight gain[g
m], W : weight gain[g cm
cm ], kp : parabolic rate constant[g
], kp : parabolic rate constant[g
 cm
cm
 s
s ], t :time[sec]
], t :time[sec]  : constant[g
: constant[g
 cm
cm
 S
S ], Q : activation energy[J・mol
], Q : activation energy[J・mol ], R : gas constant[8.314J
], R : gas constant[8.314J  K
K
 mol
mol ], T : temperature[K] (1) PE16 : kp = 1.090
], T : temperature[K] (1) PE16 : kp = 1.090 10
10 exp(-192,500/(RD)), (2) 20Cr/25Ni/Nb : kp = 1.651
exp(-192,500/(RD)), (2) 20Cr/25Ni/Nb : kp = 1.651 10
10 exp(-201,300/(RT)) High Ni alloy (JNC), (3)18Cr-8Ni : kp = 1.503
exp(-201,300/(RT)) High Ni alloy (JNC), (3)18Cr-8Ni : kp = 1.503 10
10 exp(-60,000/(RT)), (4) PNC316, PNC1520 : kp = 1.503
exp(-60,000/(RT)), (4) PNC316, PNC1520 : kp = 1.503 10
10 exp(-60,000/(RT))
exp(-60,000/(RT)) 0.62
0.62 14Cr-25Ni(JNC) The weight gain is (3)
14Cr-25Ni(JNC) The weight gain is (3)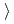 (4)
(4)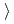 (2)
(2)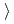 (1) in order.
(1) in order.
Honda, Takashi*; *
JNC TJ8400 2000-007, 200 Pages, 2000/02
In general, it is very difficult to evaluate the residual state of metallic iron and the original shape of iron-base archaeological artifacts, as these are covered by thick oxide films formed in the ground during over several hundred years. The purpose of this research is to quantify the corrosion of an artifact such as base, knife, and nail, which was digged out of the relics about 500-1,000 years old. (1)The outer oxide film layer and the inner metallic iron can be quantitatively divided by using X-ray CT method. Furthermore, the original surfaces of artifacts can be estimated from the obtained images, even if the metallic iron has corroded completely. The X-ray CT images were also compared with those obtained by X-ray transmission inspection. (2)The corrosion amounts and rates were evaluated on the basis of thicknesses, densities, and iron concentrations of oxide films. (3)The characteristic differences between ancient iron and modern carbon steel were evaluated by analyzing the ancient iron slag.
*; *; Tanai, Kenji
JNC TN8400 99-047, 54 Pages, 1999/11
This paper reports on the design process for a carbon-steel overpack as a key component in the engineered barrier system of a deep geological repository described in the 2nd progress report. The results of the research and development regarding design requirements, configuration, manufacturing and inspection of overpack are also described. The concept of a composite overpack composed of two different materials is also considered. First, the design requirements for an overpack and presume environmental and design conditions for a repository are provided. For a candidate material of carbon steel overpack, forging material is selected considering enough experience of using this material in nuclear power boilers and other components. Second, loading conditions after emplacement in a repository are set and the pressure-resistant thickness of overpack is calculated. The corrosion thickness to achieve an assigned 1000 year life time and the required thickness to prevent radiolysis of ground water which might enhance corrosion rate are also determined. As aresult, the total required thickness of a carbon-steel overpack is conservatively estimated to 190 mm. This is a reduction of about 30% from the previous estimate provided in the 1st Progress Report. Additional items that must be considered in manufacturring and operating overpacks (i.e. sealing of vitrified waste, examination of main body and sealing welding, mechanism of handling) are evaluated on the basis of current technology, specific future data needs are identified. With respect to the concept of composite overpack (i.e., an outer vessel to provide corrosion-allowance or corrosion-resistant performance and an inner vessel to provide pressure-resistance), the differences in design concepts between the carbon-steel overpack and such composite overpacks are analyzed. Future data needs and analytical capabilities with respect to overpacks are also summarized.
 O
O -NaOH System)
-NaOH System)Yoshida, Eiichi; Aoto, Kazumi; Hirakawa, Yasushi;
JNC TN9400 2000-024, 42 Pages, 1999/10
For the purpose of improving the reliability of evaluation, the corrosion rate equation of the carbon steel SM400B (JIS G3106) in the high-temperature sodium compounds (NaOH-Na 0
0 system) was revised. ln this revision, the data acquired after 1997 was used. Based on the experimental results, the evaluation was made to be an approach to the following; (1)Metal loss of carbon steel in NaOH-Na
system) was revised. ln this revision, the data acquired after 1997 was used. Based on the experimental results, the evaluation was made to be an approach to the following; (1)Metal loss of carbon steel in NaOH-Na 0
0 system was evaluated as increases in exposure to the time, which is linear rate law. (2)There were no significant effects of the experiment atmosphere and mixing speed of the reagent on corrosion rate. (3)The concentration of Na
system was evaluated as increases in exposure to the time, which is linear rate law. (2)There were no significant effects of the experiment atmosphere and mixing speed of the reagent on corrosion rate. (3)The concentration of Na 0
0 in sodium compound is considered for the evaluation. The concentration under experiment is made to be the over concentration necessary for maintaining the dominant reaction between Fe and Na
in sodium compound is considered for the evaluation. The concentration under experiment is made to be the over concentration necessary for maintaining the dominant reaction between Fe and Na 20
20 . As a result of the evaluation, the additional data are 67 points. The data for the revision of the evaluation equation became the total of 105 points, when existing data of 38 points were added. The statistical evaluation of 105 points was carried out, and following recommended equation was obtained. C
. As a result of the evaluation, the additional data are 67 points. The data for the revision of the evaluation equation became the total of 105 points, when existing data of 38 points were added. The statistical evaluation of 105 points was carried out, and following recommended equation was obtained. C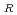 = C exp(-Q/RT) Where; C
= C exp(-Q/RT) Where; C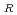 : Corrosion rate, mm/h C : Material constant Q : Apparent activation energy, cal/mol R : Gas constant, 1.986 cal/mol K T ; Absolute temperature, K Q = 9.61 kcal/mol C = 148.29 (average), 262.11 (99% UCL), 83.90 (99% LCL)
: Corrosion rate, mm/h C : Material constant Q : Apparent activation energy, cal/mol R : Gas constant, 1.986 cal/mol K T ; Absolute temperature, K Q = 9.61 kcal/mol C = 148.29 (average), 262.11 (99% UCL), 83.90 (99% LCL)
Ogawa, Masuro; Takatsu, Hideyuki; Iida, Hiromasa; Seki, Yasushi
JAERI-M 91-119, 27 Pages, 1991/08
no abstracts in English
; ; ; *
JAERI-M 82-089, 23 Pages, 1982/07
no abstracts in English